Renzzo Studio – компания, которую особенно ценят за индивидуальный подход к каждому заказчику. Мы не просто слепо следуем трендам, а учитываем пожелания клиента. Наша компания поможет сделать любое помещение уникальным, соответствующим ритму жизни, привычкам и требованиям заказчика. Думаете кому доверить дизайн интерьера Алматы? Смело обращайтесь к нам!
Команда высококвалифицированных дизайнеров, архитекторов, инженеров и визуализаторов воплощает в жизнь даже самые смелые проекты.
Мы с особым трепетом относимся к каждому проекту. Наша цель – обеспечить максимальный комфорт для заказчика. Renzzo Studio позволит вам забыть о решении технических и организационных вопросов. Задача заказчика – выразить свои пожелания, согласовать план работ и принять готовый проект. Остальное мы возьмем на себя!
Наши сотрудники учтут все ваши пожелания и помогут сделать так, чтобы ваш дом стал полным отражением вас. А качественные материалы и профессиональные работники позволят вам наслаждаться новым интерьером долгие годы.
Renzzo Studio поможет вам начать жить комфортно и красиво. Мы создаем не только уникальные, но еще и функциональные дизайны.
Renzzo Studio – это не про шаблонные дизайны. Мы создаем действительно уникальные интерьеры, которые радуют заказчиков долгие годы. Каждый реализованный нами проект – это уникальная история. История, которая удивляет разум своими деталями и особенностями.
На этом этапе происходит встреча с клиентом и обсуждение пожеланий и требований. После чего – проводится фотосъемка объекта и замеры. По итогу – составляется подробное техническое задание проекта.

Специалисты из нашей команды предложат Вам несколько вариантов планировочных решений. Это дает возможность сделать дизайн интерьера комнаты любого размера функциональным.
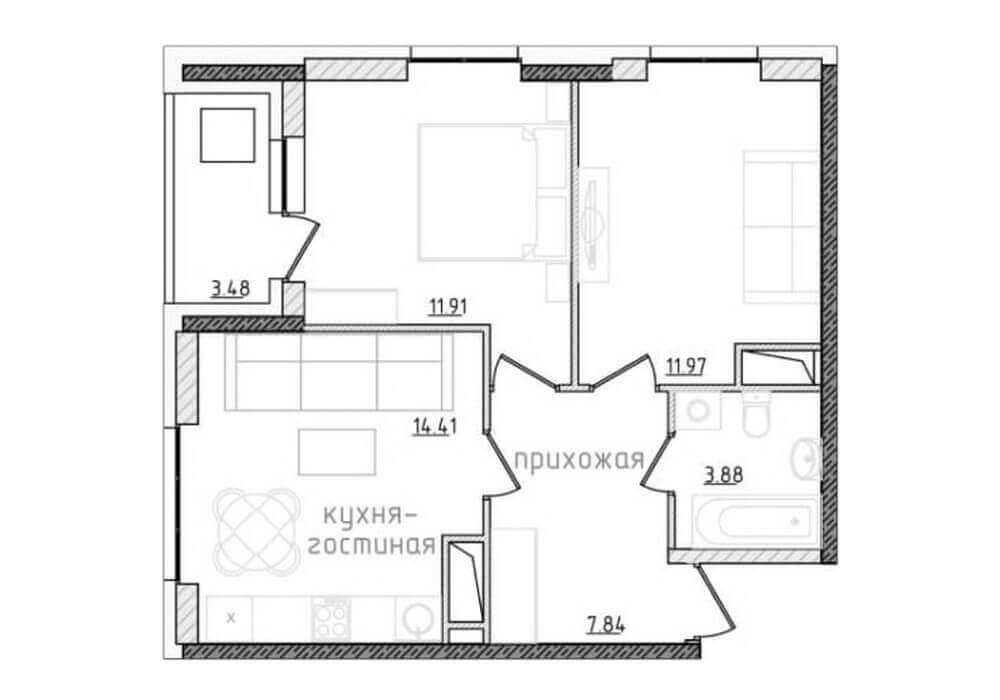
Вы сможете увидеть реалистичные изображения вашего будущего интерьера с точностью до мелочей.

Чертежи, на которых можно просмотреть все нюансы с точностью до миллиметра – предоставляются заказчику нашими специалистами. На этом этапе можно максимально точно определить объем нужных материалов, а также их стоимость.
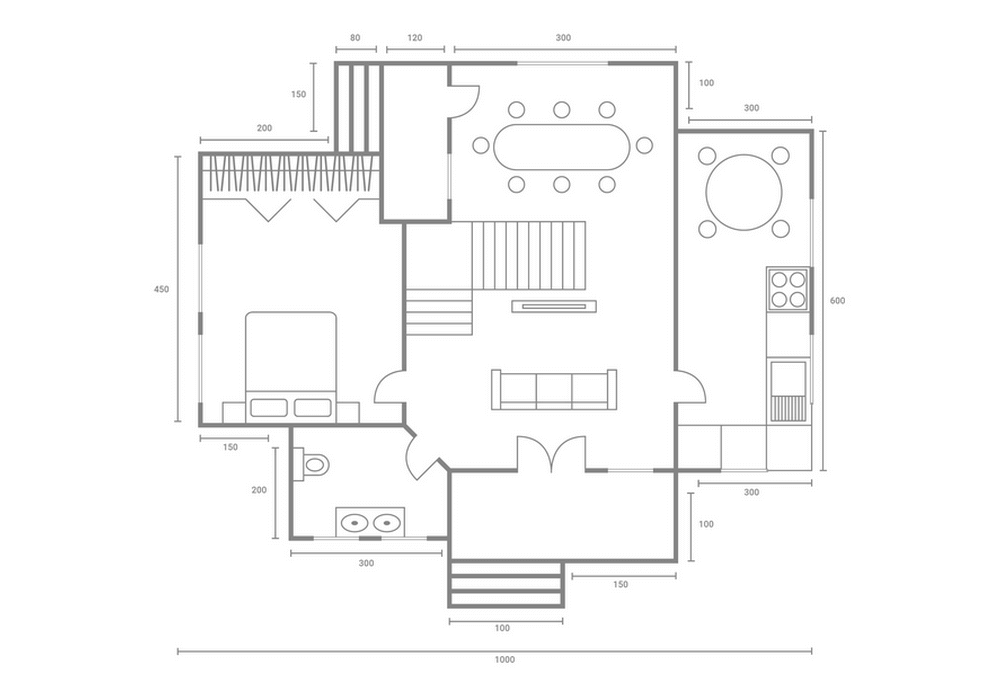
Мы сами проконтролируем качество и сроки проведения работ. Вам не нужно беспокоиться о соответствии материалов и мебели спецификациям. Дизайнер Renzzo Studio самостоятельно проведет авторский надзор.
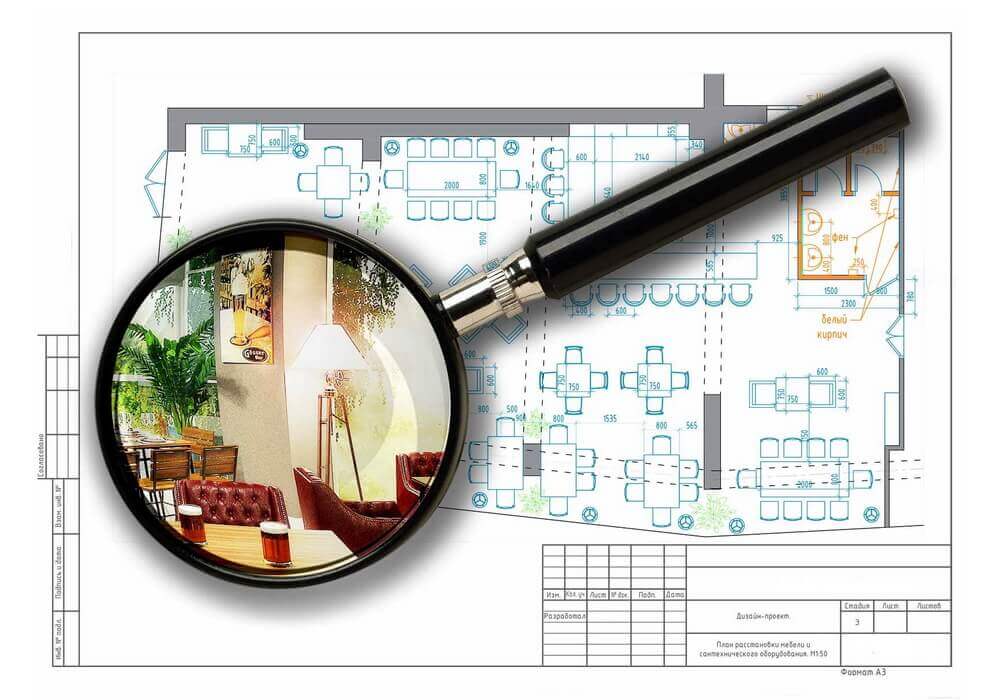
На этом этапе вы становитесь владельцем нового, современного помещения, которое полностью соответствует всем вашим пожеланиям.


Профессиональные сотрудники Renzzo Studio помогут Вам рассчитать точную стоимость проекта с учетом запланированного бюджета и потребностей. Мы за то, чтобы сделать комфортное и стильное жилье доступным каждому!
Эскизный проект от компании Renzzo Studio включает в себя замеры помещения, планировку, создание 3D-визуализации и консультацию по подбору оборудования, материалов, мебели.
Полный дизайн проект интерьера квартиры от компании Renzzo Studio включает в себя не только Эскизный проект. Помимо этого, сюда входят еще: планы демонтажных и монтажных работ; схемы расположения сантехники, розеток и выключателей; спецификации материалов и мебели; планы стен, потолков и пола.
Мы всегда ориентируемся на тренды в дизайне, чтобы создавать современные интерьеры, которые не
потеряют свою актуальность еще долгое время.
Вы всегда можете рассказать нам о своих идеях и пожеланиях, которые мы обязательно учтем при
разработке дизайн проекта.
Приватизация жилья и любые сделки с помещением без специального разрешения для перепланировки принесут ряд трудностей. Все наши проекты разрабатываются специалистами с учетом Правил перепланировки квартир в Алматы, ГОСТ и СНиП. В стоимость проекта перепланировка не входит.
Политика конфиденциальности
Администрация сайта (далее Сайт) с уважением относится к правам посетителей Сайта. Мы безоговорочно
признаем важность конфиденциальности личной информации посетителей Сайта. Данная страница содержит
сведения о том, какую информацию мы получаем и собираем, когда Вы пользуетесь Сайтом. Мы надеемся, что
эти сведения помогут Вам принять осознанное решение в отношении предоставляемой нам личной информации.
Настоящая Политика конфиденциальности распространяется только на Сайт и информацию, собираемую данным
сайтом и посредством него. Она не распространяется ни на какие другие сайты и не применима к веб-сайтам
третьих лиц, которые могут ссылаться на данный Сайт.
Получаемые сведения
Сведения, которые мы получаем на Сайте, могут быть использованы только для того, чтобы облегчить Вам
пользование Сайтом. Сайт собирает только личную информацию, которую Вы предоставляете добровольно при
посещении или регистрации на Сайте. Понятие «личная информация» включает информацию, которая определяет
Вас как конкретное лицо, например, Ваше имя или адрес электронной почты или телефон. Совместное
использование информации Администрация Сайта ни при каких обстоятельствах не продает и не передает в
пользование Вашу личную информацию, каким бы то ни было третьим сторонам. Мы также не раскрываем
предоставленную Вами личную информацию за исключением случаев предусмотренных законодательством РФ.
Отказ от ответственности
Помните, передача информации личного характера при посещении сторонних сайтов, включая сайты
компаний-партнеров, даже если веб-сайт содержит ссылку на Сайт или на Сайт есть ссылка на эти веб-сайты,
не подпадает под действия данного документа. Администрация Сайта не несет ответственности за действия
других веб-сайтов. Процесс сбора и передачи информации личного характера при посещении этих сайтов
регламентируется документом «Защита информации личного характера» или аналогичным, расположенном на
сайтах этих компаний.